Example
Mineral traps greenhouse gases
Background
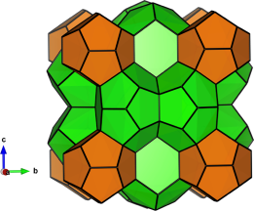
Melanophlogite is one of naturally occurring SiO2 minerals, and has a clathrate structure. It has relatively large cavities (two cages) in the structure, and can accommodate CO2, methane, nitrogen molecules etc. It has been suggested that melanophlogite can be used to isolate and store CO2 and methane which are two most important greenhouse gases.
Purpose
In order to use melanophlogite for sequestration and storage purposes, we need to understand high-temperature stability of CO2 and its behavior in melanophlogite structure. For that end, we are conducting present research.
Results obtained so far
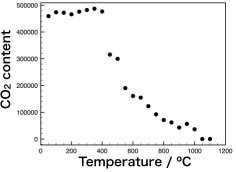
Degassing behavior of natural CO2-containing melanophlogite has been studied by in-situ Raman spectroscopy. We found that degassing of CO2 starts from 450 ºC, and melanophlogite itself is stable up to 1000 ºC. We also obtained valuable information regarding diffusion process of CO2.
Expected outcome
Our study may be used to judge whether this mineral is practical to use for sequestration and storage purposes.
URL
http://www.misasa.okayama-u.ac.jp/~masami/
Representative
